CIICA AT ESPCI, HANNOVER

ESPCI began in 1992 in Nottingham, led by Gerry O’Donoghue, Mark Lutman and Sue Archbold, with 350 delegates from across the globe. Gerry received the lifetime award for services to CI from Prof Thomas Lenarz, for his outstanding contribution to the field.
ESPCI, 2025 began with a lecture organised by Professor Monika Lehnhardt on the history of multi-channel CI in Europe, as a tribute to her late husband, Prof Ernst Lehnhardt. A group of the early pioneers contributed to the fascinating session which brought together the developments since the 70’s and 80’s.

The audience were inspired to hear about the challenges that had been overcome over the years and Sue Archbold of CIICA contributed her memories of being the teacher of the deaf of the first child to have a CI in the UK and her abrupt transition from the classroom to managing a rapidly growing multi disciplinary CI programme.
She commented that she felt teachers hadn’t kept up with the huge changes and impact that CI has had on the education of deaf children.


Throughout the conference, the CIICA stand was well visited, and collaborated with EURO-CIU, with President Tobias Fischer, and the Lenhardt Foundation, and AVID books with Tanya Saunders. It was great to share with so many our plans for our conference in October, and to attract new members. Leo De Raeve led a popular session on Lifelong Services for Children with CI, and Leo and Harald Seidler took part in a session led by Sue entitled Nothing About us Without Us. You can find the presentations of this group pictured here:

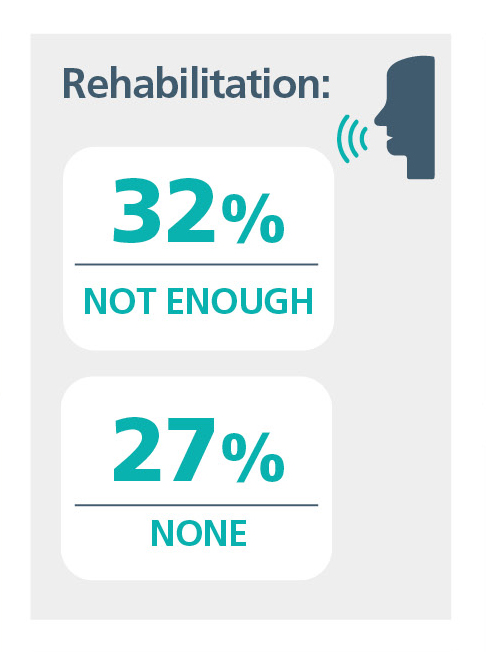
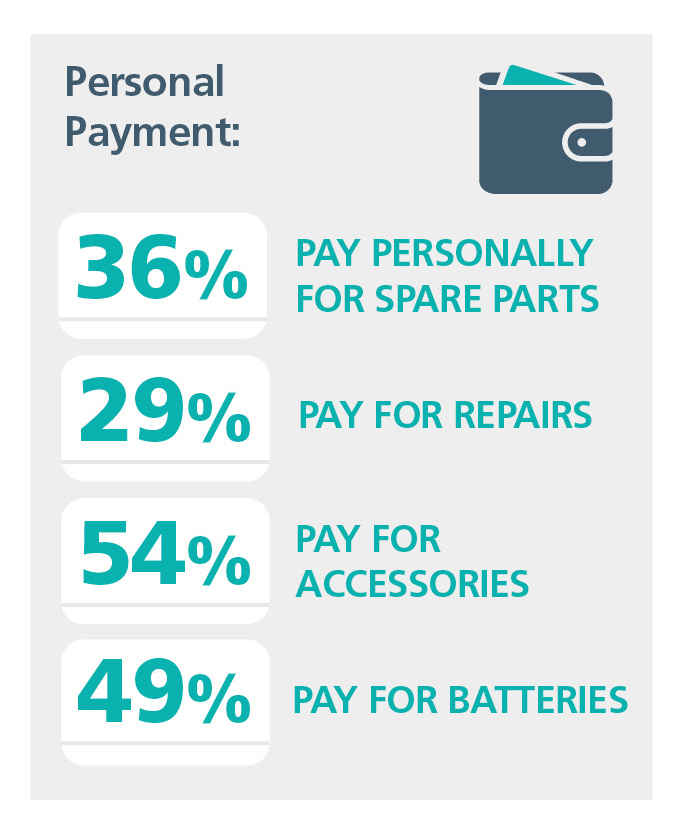
On the final morning, Sue presented her plenary session on the challenges of providing sustainable lifelong services and questioned, as had Monika Lehnhardt, as to whether CI was in a period of stagnation, and she asked some challenging questions as you can see. This provoked some great feedback with people keen to ensure we can look to a sustainable future.
A collection of photographs capturing CIICA at the conference with friends and colleagues from The Lehnhardt Foundation, EURO-CIU, AVID books.